The initial aim for the initiative was to procure and implement a digital archive that ensures records from Barts Health clinical trials are preserved, accessible and usable for their whole retention period (mainly 25 years). It needed to be a specialist archive because research records must be retained according to strict regulations that can be challenging to meet. With Arkivum’s system implemented in 2023, the aim is to now extend the service to other NHS Trusts who cannot procure a repository themselves. The team at Barts Health NHS Trust wants to help preserve data within healthcare research and raise awareness of digital preservation in this area. Research records are unusual in that they have lengthy retention periods and require long-term preservation, but they are not always transferred to the archive for permanent preservation afterwards.
A risk-based approach was taken for procuring the system, as not having an approved digital archive created a risk at Trust level. The action plan to mitigate the risk was procuring a suitable system, and so executive approval was gained because the risk of not having one in place was so high. The methodology for extending the service to other NHS Trusts is so far effective (the service is to be fully set up by June/July). There is a defined process for setting up commercial ventures in our organisation, which is being followed, and the team has been working closely with Arkivum to design the service.
Barts is at the forefront of digital preservation for research study records. This is not something widely considered yet. Organisations that are aware of its importance have not started tackling the challenge as they are uncomfortable with the demands and do not feel adequately equipped. And there are no guidelines or examples to follow as this has not been done in the NHS before; national research guidance for records storage is still largely based on paper. Not only has Barts been able to procure a digital archive that meets research standards, but developing this further to provide digital preservation to other Trusts is unique.
Having a research-approved digital archive provides the confidence and protection research teams require. It ensures the information from a study is preserved, accessible and usable for the whole retention period and guarantees the records are inspection ready. Research teams and sponsors benefit by not having to worry about what they are going to do with the records at study close and patients ultimately benefit because the availability of data supports advances in healthcare. This initiative is timely: the Society for Clinical Research Sites predicts that records retention will be a popular topic in the coming years. 70% of their survey respondents last year said they want to refrain from offering long-term document storage as it is seen as burdensome. Barts and Arkivum are offering a solution to that which will hopefully encourage and enable records management in the sector to grow and evolve.
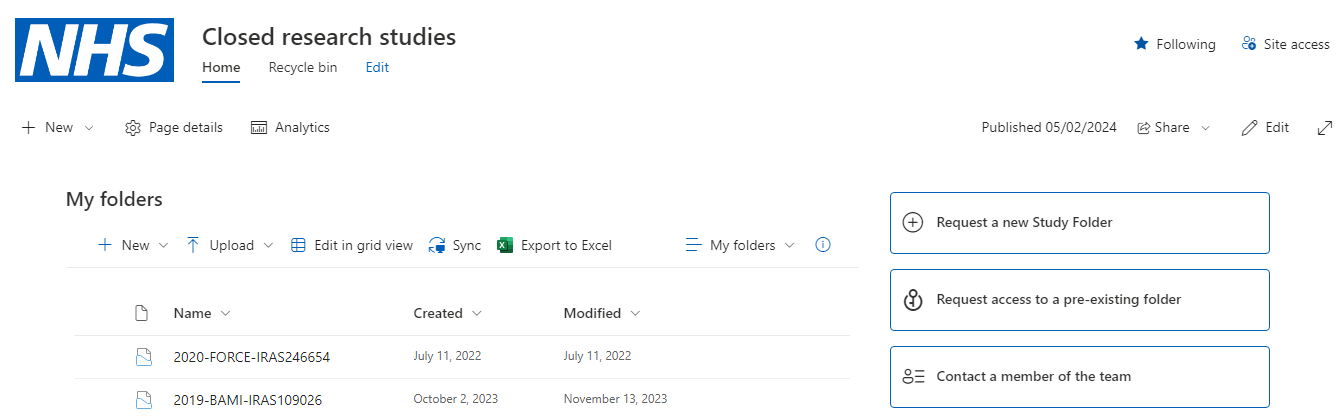
Much determination and resources were spent on implementing the digital archive for Barts. The first three months were spent developing a bespoke SharePoint site; research teams themselves had made it known how urgently they needed to start storing digital records as well as paper. The SharePoint site was created as an interim solution, so the records were at least safe and secure until the specialist archive was implemented. There was a financial cost as well as time and physical work. Then for approximately one year digital preservation vendors were consulted, quotes were considered, a risk assessment was completed and submitted, and the Finance team was consulted before a feasibility proposal was submitted. This was challenging without any examples of this having previously been done for guidance. There were one-off costs for the onboarding process and there is an annual subscription for the software. Once procured, several months were then spent receiving training and implementing the system. For extending the service to other Trusts, many hours have been spent working with the Barts Commercial team and Arkivum; to calculate financials, agree on contract changes, write new Service Level Agreements (SLAs) and put new processes together. National guidance on records storage fees in research is still based on boxes of paper, so costings and fees were calculated with assistance from the Commercial team.
The Information Governance Committee and Joint Research Management Office are very happy and supportive of the work. The Trust Risk Manager wants to use this work as a case study. The Trust Archives holds one of the UK's largest collections of hospital and healthcare records, so the Archive team is pleased to be able to also use the software to preserve their digital collections.
Records such as the Trial Master File (TMF) prove that a research study has been conducted according to regulatory requirements. The TMF plays a vital role in ensuring that the study has been managed successfully and allows it to be reconstructed and evaluated if necessary. This enables public trust in research being conducted ethically and appropriately.
Research plays a vital role in the NHS and preserving research records enables the knowledge gained from studies to enhance care, not only for Trust patients, but for whole populations.





























































































































Planning ahead for DVD-Video migration research
Starting with complexity: Archiving digital-born music compositions from Mac systems of the 80s/90s